A COMPARISON OF AUTOMATED COMPLETE BLOOD COUNT PARAMETERS AND HEMOLYSIS OCCURRENCE FROM VACUUM AND NON-VACUUM K2EDTA TUBES
DOI:
https://doi.org/10.55374/jseamed.v9.235Keywords:
vacuum K₂EDTA tubes, non-vacuum K₂EDTA tubes, under-filled collection tubes, CBC analysis, hemolysis levelAbstract
Background: The preanalytical factors, including tube type and storage duration, may influence CBC results and hemolysis. Nowadays, blood collection using K₂EDTA, both vacuum and non-vacuum tubes, is widely utilized in clinical laboratories for complete blood count (CBC) analysis. However, the impact of vacuum-induced negative pressure and the anticoagulant-to-blood ratio in under-filled tubes on sample integrity remains a concern.
Objective: This study aimed to compare the performance of K₂EDTA vacuum and non-vacuum blood collection tubes, including under-filled non-vacuum tubes, in automated CBC parameters and to assess hemolysis occurrence at different time points.
Methods: Blood samples from 30 healthy subjects were collected into three different K₂EDTA blood collection tubes: vacuum, non-vacuum, and non-vacuum tubes with under-filled volumes. CBC parameters, including RBC, Hb, Hct, MCV, MCH, MCHC, RDW, WBC, PLT, and MPV, were analyzed at 0 hours, 3 hours, and 24 hours post-collection. Hemolysis was assessed via spectrophotometry at 540 nm.
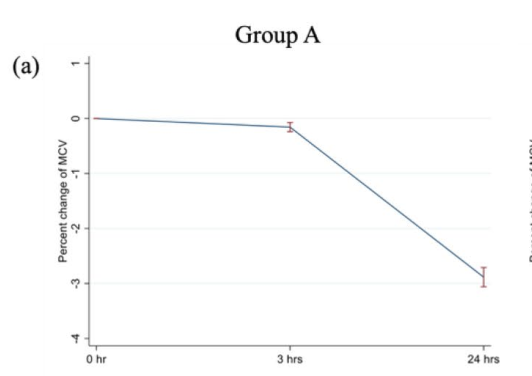
Results: No statistically significant differences were observed in CBC parameters among the three groups at 0 hours. However, significant differences occurred at 3 hours post-collection, including WBC and PLT in vacuum tubes, MCV in non-vacuum tubes, RDW in under-filled tubes, and MPV in all tube types. At 24 hours, all tube types showed significant changes in MCV, MCHC, RDW, and MPV, while RBC showed only changes in vacuum tubes. The highest hemolysis levels occurred in vacuum tubes at 0 hours, whereas under-filled tubes exhibited increased hemolysis at 3 and 24 hours.
Conclusion: The results indicated that all three tube types are suitable for hematological analysis if processed within 3 hours. However, the risk of hemolysis in vacuum tubes should be carefully considered, particularly in individuals with suspected hemolysis.
Downloads
Metrics
References
Adcock Funk DM, Lippi G, Favaloro EJ. Quality standards for sample processing, transportation, and storage in hemostasis testing. Semin Thromb Hemost. 2012; 38: 576-85. DOI: https://doi.org/10.1055/s-0032-1319768
Zahraini H, Indrasari YN, Kahar H. Comparison of K2 and K3 EDTA anticoagulant on complete blood count and erythrocyte sedimentation rate. Indonesian J Clin Pathol Med Lab. 2021; 28: 75–9. DOI: https://doi.org/10.24293/ijcpml.v28i1.1735
Mehmood R, Muhammed RK, Hussain S, Sana A. Evaluation of di-potassium and tri-potassium EDTA evacuated tubes for routine haematological testing. J Clin Lab Anal. 2018; 32: e22188. DOI: https://doi.org/10.1002/jcla.22188
World Health Organization. WHO guidelines on drawing blood: best practices in phlebotomy. Geneva: World Health Organization; 2010.
Clinical and Laboratory Standards Institute. Collection of diagnostic venous blood specimens. 7th ed. CLSI standard GP41. Wayne, PA: Clinical and Laboratory Standards Institute; 2017.
International Organization for Standardization. ISO 15189:2022. Medical laboratoriesrequirements for quality and competence. Geneva: ISO; 2022.
Weikart CM, Breeland AP, Wills MS, Baltazar-Lopez ME. Hybrid blood collection tubes: combining the best attributes of glass and plastic for safety and shelf life. SLAS Technol. 2020; 25: 484-93. DOI: https://doi.org/10.1177/2472630320915842
Hu J, Zhang QX, Xiao TT, Pan MC, Cai YM. Residual negative pressure in vacuum blood-collection tube and hemolysis in pediatric blood specimens. Lab Med. 2020; 51: 41-6. DOI: https://doi.org/10.1093/labmed/lmz026
Guven B, Benice I, Can M. Undefilled blood tube containing EDTA: Is it an inappropriate sample for HbA1c assay? Biochem Med (Zagreb). 2023; 33: 010901 DOI: https://doi.org/10.11613/BM.2023.010901
Lippi G, Dima F, Tosi M, Carpenè G, Celegon G, Favaloro EJ, el at. Incomplete filling of spray-dried K2EDTA evacuated blood tubes: impact on measuring routine hematological parameters on Sysmex XN-10. Diagnosis. 2023; 10: 440-5. DOI: https://doi.org/10.1515/dx-2023-0064
Neuwinger N, Meyer Zum Büschenfelde D, Tauber R, Kappert K. Underfilling of vacuum blood collection tubes leads to increased lactate dehydrogenase activity in serum and heparin plasma samples. Clin Chem Lab Med. 2020; 58: 213-21. DOI: https://doi.org/10.1515/cclm-2019-0616
Rosada A, Prpic M, Spieß E, Friedrich K, Neuwinger N, Müller-Werdan U, et al. Underfilled blood collection tubes as pathologizing factor for measured laboratory parameters in older patients. J Am Geriatr Soc. 2024; 72:1553-6. DOI: https://doi.org/10.1111/jgs.18790
Benati M, Pighi L, Paviati E, Visconti S, Lippi G, Salvagno GL. Preanalytical impact of incomplete K2EDTA blood tube filling in molecular biology testing. Diagnostics. 2024; 14: 1934. DOI: https://doi.org/10.3390/diagnostics14171934
Simundic AM, Baird G, Cadamuro J, Costelloe SJ, Lippi G. Managing hemolyzed samples in clinical laboratories. Crit Rev Clin Lab Sci. 2020; 57: 1-21. DOI: https://doi.org/10.1080/10408363.2019.1664391
Mrazek C, Simundic AM, Wiedemann H, Krahmer F, Felder TK, Kipman U, et al. The relationship between vacuum and hemolysis during catheter blood collection: a retrospective analysis of six large cohorts. Clin Chem Lab Med. 2017; 55:1129-34. DOI: https://doi.org/10.1515/cclm-2016-0940
Kazezoglu C, Serin E. The effect of different blood drawing methods on hemolysis and test results from intravenous catheters used in emergency departments. Clin Lab. 2019; 65. DOI: https://doi.org/10.7754/Clin.Lab.2018.180614
Lippi G, Avanzini P, Musa R, Sandei F, Aloe R, Cervellin G. Evaluation of sample hemolysis in blood collected by S-Monovette using vacuum or aspiration mode. Biochem Med (Zagreb). 2013; 23: 64-9. DOI: https://doi.org/10.11613/BM.2013.008
Srimahadthai S. Comparison of rate of hemolysis between vacuum blood collection tube versus syringe method. Reg 4-5 Med J. 2014; 33: 281-9.
Heireman L, Van Geel P, Musger L, Heylen E, Uyttenbroeck W, Mahieu B. Causes, consequences and management of sample hemolysis in the clinical laboratory. Clin Biochem. 2017; 50: 1317-22. DOI: https://doi.org/10.1016/j.clinbiochem.2017.09.013
Gunawardena D, Jayaweera S, Madhubhashini G, Lokumarakkala DD, Senanayake SJ. Reliability of parameters of complete blood count with different storage conditions. J Clin Lab Anal. 2017; 31: e22042. DOI: https://doi.org/10.1002/jcla.22042
Schapkaitz E, Pillay D. Prolonged storage-induced changes in haematology parameters referred for testing. Afr J Lab Med. 2015; 4: 208. DOI: https://doi.org/10.4102/ajlm.v4i1.208
Antwi-Baffour S, Quao E, Kyeremeh R, Mahmood SA. Prolong storage of blood in EDTA has an effect on the morphology and osmotic fragility of erythrocytes. Int J Biomed Sci Eng. 2014; 1: 20-3. DOI: https://doi.org/10.11648/j.ijbse.20130102.11
Wu DW, Li YM, Wang F. How long can we store blood samples: a systematic review and meta-analysis. EBioMedicine. 2017; 24: 277-85. DOI: https://doi.org/10.1016/j.ebiom.2017.09.024
Doeleman MJH, Esseveld A, Huisman A, de Roock S, Tiel Groenestege WM. Stability and comparison of complete blood count parameters between capillary and venous blood samples. Int J Lab Hematol. 2023; 45: 659-67. DOI: https://doi.org/10.1111/ijlh.14080
Åstrand A, Wingren C, Walton C, Mattsson J, Agrawal K, Lindqvist M, et al. A comparative study of blood cell count in four automated hematology analyzers: An evaluation of the impact of preanalytical factors. PLoS One. 2024; 19: e0301845. DOI: https://doi.org/10.1371/journal.pone.0301845
Bowen RAR, Remaley AT. Interferences from blood collection tube components on clinical chemistry assays. Biochem Med (Zagreb). 2014; 24: 31-44. DOI: https://doi.org/10.11613/BM.2014.006
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Journal of Southeast Asian Medical Research

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
The Journal of Southeast Asian Medical Research will hold the copyright to all published articles. The publisher's production department handles copyright forms once a manuscript is accepted and scheduled for publication.







