A COMPARISON OF YIELD AND QUALITY OF CIRCULATING CELL-FREE DNA FROM K2EDTA AND K3EDTA COLLECTIONS IN HEALTHY SUBJECTS
DOI:
https://doi.org/10.55374/jseamed.v8.231Keywords:
Cell-free DNA, K2EDTA, K3EDTA, Anticoagulant tubes, Molecular diagnosticsAbstract
Background: Circulating cell-free DNA (cfDNA) is a biomarker for various clinical applications, including detecting and monitoring cancer. However, blood collection tubes can affect the yield and quality of cfDNA. Since specific cfDNA collection tubes are costly, K2EDTA and K3EDTA anticoagulant tubes are alternatives in routine clinical laboratories.
Objectives: This study aimed to compare the efficiency of cfDNA extraction from plasma collected in K2EDTA and K3EDTA tubes and evaluate implementation for molecular diagnostics.
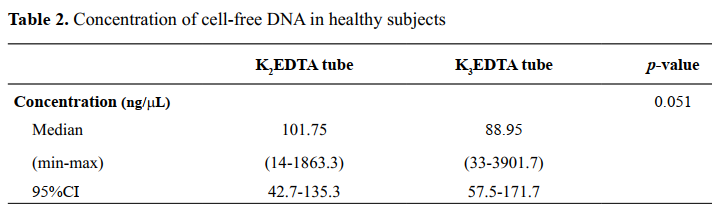
Methods: Blood samples from 38 healthy subjects were collected in K2EDTA and K3EDTA tubes that were processed within 2 hours. The extracted cfDNA was measured and performed using SYBR Green-based qPCR for three endogenous reference genes (GAPDH, HPRT1, TFRC). The cfDNA yield and the amplification efficiency of these genes were compared between K2EDTA and K3EDTA tubes using the Mann-Whitney U test. Results: There were no significant differences in cfDNA concentration between K2EDTA and K3EDTA tubes (p=0.051). However, qPCR analysis revealed significantly higher copy numbers of TFRC and HPRT1 in K2EDTA tubes than in K3EDTA tubes (p<0.05). No significant difference was found for GAPDH.
Conclusion: The results indicate that K2EDTA and K3EDTA tubes are an alternative option for cfDNA analysis if samples are processed quickly after a blood draw, which offers flexibility and cost savings in resource-limited areas.
Downloads
Metrics
References
Stroun M, Lyautey J, Lederrey C, Olson-Sand A, Anker P. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin Chim Acta 2001; 313: 139-42. DOI: https://doi.org/10.1016/S0009-8981(01)00665-9
Schwarzenbach H, Hoon DS, Pantel K. Cellfree nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011; 11: 426-37. DOI: https://doi.org/10.1038/nrc3066
Kustanovich A, Schwartz R, Peretz T, Grinshpun A. Life and death of circulating cell-free DNA. Cancer Biol Ther 2019; 20: 1057-67. DOI: https://doi.org/10.1080/15384047.2019.1598759
Schwarzenbach H, Pantel K. Circulating DNA as biomarker in breast cancer. Breast Cancer Res 2015; 17: 136. DOI: https://doi.org/10.1186/s13058-015-0645-5
Xu J, Gao H, Guan X, Meng J, Ding S, Long Q, et al. Circulating tumor DNA: from discovery to clinical application in breast cancer. Front Immunol 2024; 15: 1355887. DOI: https://doi.org/10.3389/fimmu.2024.1355887
Khurram I, Khan MU, Ibrahim S, Saleem A, Khan Z, Mubeen M, et al. Efficacy of cellfree DNA as a diagnostic biomarker in breast cancer patients. Sci Rep 2023; 13: 15347. DOI: https://doi.org/10.1038/s41598-023-42726-6
Butler TM, Spellman PT, Gray J. Circulatingtumor DNA as an early detection and diagnostic tool. Curr Opin Genet Dev 2017; 42: 14-21. DOI: https://doi.org/10.1016/j.gde.2016.12.003
Xue R, Yang L, Yang M, Xue F, Li L, Liu M, et al. Circulating cell-free DNA sequencing for early detection of lung cancer. Expert Rev Mol Diagn 2023; 23: 589-606. DOI: https://doi.org/10.1080/14737159.2023.2224504
P P, Keshari JR, Prakash P, Kumar M, Mandal M, Kumari R. Correlation between circulating cell-free DNA levels and breast cancer subtypes: A prospective observational study. Cureus 2023; 15: e42247. DOI: https://doi.org/10.7759/cureus.42247
Meddeb R, Pisareva E, Thierry AR. Guidelines for the pre-analytical conditions for analyzing circulating cell-free DNA. Clin Chem 2019; 65: 623-33. DOI: https://doi.org/10.1373/clinchem.2018.298323
Parpart-Li S, Bartlett B, Popoli M, Adleff V, Tucker L, Steinberg R, et al. The effect of preservative and temperature on the analysis of circulating tumor DNA. Clin Cancer Res 2017; 23: 2471-77. DOI: https://doi.org/10.1158/1078-0432.CCR-16-1691
Diaz IM, Nocon A, Held SAE, Kobilay M, Skowasch D, Bronkhorst AJ, et al. Pre-analytical evaluation of Streck cell-free DNA blood collection tubes for liquid profiling in oncology. Diagnostics (Basel) 2023; 13: 1288. DOI: https://doi.org/10.3390/diagnostics13071288
Mehrotra M, Singh RR, Chen W, Huang RSP, Almohammedsalim AA, Barkoh BA, et al. Study of preanalytic and analytic variables for clinical next-generation sequencing of circulating cell-free nucleic acid. J Mol Diagn 2017; 19: 514-24. DOI: https://doi.org/10.1016/j.jmoldx.2017.03.003
Risberg B, Tsui DWY, Biggs H, Ruiz-Valdepenas Martin de Almagro A, Dawson SJ, et al. Effects of collection and processing procedures on plasma circulating cell-free DNA from cancer patients. J Mol Diagn 2018; 20: 883-92. DOI: https://doi.org/10.1016/j.jmoldx.2018.07.005
Xue X, Teare MD, Holen I, Zhu YM, Woll PJ. Optimizing the yield and utility of circulating cell-free DNA from plasma and serum. Clin Chim Acta 2009; 404 : 100-4. DOI: https://doi.org/10.1016/j.cca.2009.02.018
Banfi G, Salvagno GL, Lippi G. The role of ethylenediamine tetraacetic acid (EDTA) as in vitro anticoagulant for diagnostic purposes. Clin Chem Lab Med 2007; 45: 565-76. DOI: https://doi.org/10.1515/CCLM.2007.110
Zahraini H, Indrasari YN, Kahar H. Comparison of K2 and K3 EDTA anticoagulant on complete blood count and erythrocyte sedimentation rate. Indonesian J Clin Pathol Med Lab 2021; 28: 75-9. DOI: https://doi.org/10.24293/ijcpml.v28i1.1735
Ahn S, Cho SM, Shin H, Lee KA. Comparison of Improvacuter EDTA tube with BD Vacutainer EDTA tube for routine hematological analysis: Clinical significance of differences, stability study, and effects of K2 and K3 EDTA. J Lab Med Qual Assur 2016; 38: 77-86. DOI: https://doi.org/10.15263/jlmqa.2016.38.2.77
Mehmood R, Muhammed RK, Hussain S, Sana A. Evaluation of di-potassium and tri-potassium EDTA evacuated tubes for routine haematological testing. J Clin Lab Anal 2018; 32: e22188. DOI: https://doi.org/10.1002/jcla.22188
Recommendations of the International Council for Standardization in Haematology for Ethylenediaminetetraacetic Acid Anticoagulation of Blood for Blood Cell Counting and Sizing. International Council for Standardization in Haematology: Expert Panel on Cytometry. Am J Clin Pathol 1993; 100: 371-2. DOI: https://doi.org/10.1093/ajcp/100.4.371
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Journal of Southeast Asian Medical Research

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
The Journal of Southeast Asian Medical Research will hold the copyright to all published articles. The publisher's production department handles copyright forms once a manuscript is accepted and scheduled for publication.







