EFFICACY OF DOUBLE DOSE VERSUS STANDARD DOSE ERGOCALCIFEROL ON VITAMIN D STATUS AMONG PATIENTS WITH CHRONIC KIDNEY DISEASE
DOI:
https://doi.org/10.55374/jseamed.v4i2.70Keywords:
Hyperparathyroidism, Chronic kidney disease, 25-hydroxyvitamin D deficiency, ErgocalciferolAbstract
Background: Patients with chronic kidney disease (CKD) have an exceptionally high rate of 25-hydroxyvitamin D (25-OHD) deficiency. Modest supplementation with ergocalciferol to raise serum 25-OH-D levels might improve bone and mineral disorders in CKD. Limited evidence is available regarding dosage of ergocalciferol supplement in CKD populations.
Objectives: The study aimed to examine the effectiveness of double-dose ergocalciferol on serum 25-OHD, serum intact parathyroid hormone (PTH) levels and mineral and safety profiles compared with standard-dose ergocalciferol among CKD subjects.
Methods: The study employed a 12-week open labeled, randomized, controlled design among patients with CKD at stages III-IV and serum 25-OHD <30 ng/mL. Patients were randomized in 2 groups: standard dose treated with ergocalciferol as recommended by K/DOQI guidelines or double dose of ergocalciferol from recommendations. Serum testing including 25-OHD, intact PTH, phosphate and calcium was performed at baseline and week 12.
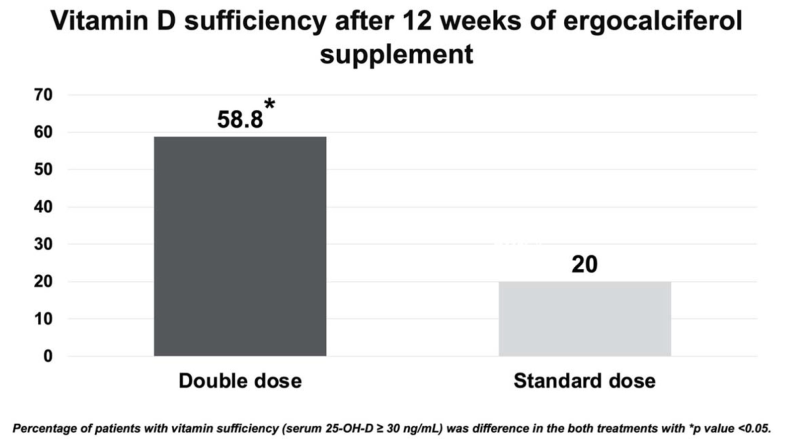
Results: Sixty-three patients were included [standard-dose group (N=30) and double-dose group (N=34)]. At the end of the 12 weeks, 20 (58.8%) patients in the double dose ergocalciferol group achieved sufficiency compared with 6 (20%) patients in the standard dose ergocalciferol group (p<0.05). A significant increase in serum 25-OHD levels (13.6±9.9 vs. 8.5±6.8 ng/mL, p=0.030) and decrease in serum PTH level group (-16.8±26.4 vs. -0.3±26.8 pg/mL, p=0.030) was found in the double-dose group compared with the standard-dose group. No adverse effect was associated with the treatment.
Conclusion: The study demonstrated that high dose oral ergocalciferol had higher efficacy to increase serum 25-OHD and decrease serum PTH levels among patients with CKD than standard-dose ergocalciferol after 12 weeks of treatment.
Downloads
Metrics
References
Yadav AK, Kumar V, Kumar V, Banerjee D, Gupta KL, Jha V. The Effect of Vitamin D Supplementation on Bone Metabolic Markers in Chronic Kidney Disease. J Bone Miner Res 2018; 33: 404-9. DOI: https://doi.org/10.1002/jbmr.3314
Gonzalez EA, Sachdeva A, Oliver DA, Martin KJ. Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am J Nephrol 2004; 24: 503-10. DOI: https://doi.org/10.1159/000081023
Jayedi A, Soltani S, Shab-Bidar S. Vitamin D status and all-cause mortality in patients with chronic kidney disease: A systematic review and dose-response meta-analysis. J Clin Endocrinol Metab 2017; 102: 2136-45. DOI: https://doi.org/10.1210/jc.2017-00105
Xie S, Huang L, Cao W, et al. Association between serum 25-hydroxyvitamin D and diabetic kidney disease in Chinese patients with type 2 diabetes. PLoS One 2019; 14: e0214728. DOI: https://doi.org/10.1371/journal.pone.0214728
Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis 2011; 58: 374-82. DOI: https://doi.org/10.1053/j.ajkd.2011.03.020
Ravani P, Malberti F, Tripepi G, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int 2009; 75: 88-95. DOI: https://doi.org/10.1038/ki.2008.501
Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71: 31-8. DOI: https://doi.org/10.1038/sj.ki.5002009
Alvarez JA, Law J, Coakley KE, et al. Highdose cholecalciferol reduces parathyroid hormone in patients with early chronic kidney disease: a pilot, randomized, doubleblind, placebo-controlled trial. Am J Clin Nutr 2012; 96: 672-9. DOI: https://doi.org/10.3945/ajcn.112.040642
Dogan E, Erkoc R, Sayarlioglu H, Soyoral Y, Dulger H. Effect of depot oral cholecalciferol treatment on secondary hyperparathyroidism in stage 3 and stage 4 chronic kidney diseases patients. Ren Fail 2008;30:407-10. DOI: https://doi.org/10.1080/08860220801964210
Alvarez J, Wasse H, Tangpricha V. Vitamin D supplementation in pre-dialysis chronic kidney disease: A systematic review. Dermatoendocrinol 2012; 4: 118-27. DOI: https://doi.org/10.4161/derm.20014
Thimachai P, Supasyndh O, Chaiprasert A, Satirapoj B. Efficacy of High vs. Conventional Ergocalciferol Dose for Increasing 25- Hydroxyvitamin D and Suppressing Parathyroid Hormone Levels in Stage III-IV CKD with Vitamin D Deficiency/Insufficiency: A Randomized Controlled Trial. J Med Assoc Thai 2015; 98: 643-8.
Alfieri C, Ruzhytska O, Vettoretti S, Caldiroli L, Cozzolino M, Messa P. Native Hypovitaminosis D in CKD Patients: From Experimental Evidence to Clinical Practice. Nutrients 2019; 11: 1918. DOI: https://doi.org/10.3390/nu11081918
Hewison M. Vitamin D and immune function: autocrine, paracrine or endocrine? Scand J Clin Lab Invest Suppl 2012; 243: 92-102.
Goldsmith DJ. Pro: Should we correct vitamin D deficiency/insufficiency in chronic kidney disease patients with inactive forms of vitamin D or just treat them with active vitamin D forms? Nephrol Dial Transplant 2016; 31: 698-705. DOI: https://doi.org/10.1093/ndt/gfw082
Lee YH, Kim JE, Roh YH, et al. The combination of vitamin D deficiency and mild to moderate chronic kidney disease is associated with low bone mineral density and deteriorated femoral microarchitecture: results from the KNHANES 2008-2011. J Clin Endocrinol Metab 2014; 99: 3879-88. DOI: https://doi.org/10.1210/jc.2013-3764
Zisman AL, Hristova M, Ho LT, Sprague SM. Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol 2007; 27: 36-43. DOI: https://doi.org/10.1159/000098561
Bhan I, Dobens D, Tamez H, et al. Nutritional vitamin D supplementation in dialysis: a randomized trial. Clin J Am Soc Nephrol 2015; 10: 611-9. DOI: https://doi.org/10.2215/CJN.06910714
Kovesdy CP, Lu JL, Malakauskas SM, Andress DL, Kalantar-Zadeh K, Ahmadzadeh S. Paricalcitol versus ergocalciferol for secondary hyperparathyroidism in CKD stages 3 and 4: a randomized controlled trial. Am J Kidney Dis 2012; 59: 58-66. DOI: https://doi.org/10.1053/j.ajkd.2011.06.027
Al-Aly Z, Qazi RA, Gonzalez EA, Zeringue A, Martin KJ. Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis 2007; 50 :59-68. DOI: https://doi.org/10.1053/j.ajkd.2007.04.010
Oksa A, Spustova V, Krivosikova Z, et al. Effects of long-term cholecalciferol supplementation on mineral metabolism and calciotropic hormones in chronic kidney disease. Kidney Blood Press Res 2008; 31: 322-9. DOI: https://doi.org/10.1159/000157177
Kandula P, Dobre M, Schold JD, Schreiber MJ, Jr., Mehrotra R, Navaneethan SD. Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol 2011; 6: 50-62. DOI: https://doi.org/10.2215/CJN.03940510
Arcidiacono MV, Yang J, Fernandez E, Dusso A. The induction of C/EBPbeta contributes to vitamin D inhibition of ADAM17 expression and parathyroid hyperplasia in kidney disease. Nephrol Dial Transplant 2015; 30: 423-33. DOI: https://doi.org/10.1093/ndt/gfu311
Satirapoj B, Limwannata P, Chaiprasert A, Supasyndh O, Choovichian P. Vitamin D insufficiency and deficiency with stages of chronic kidney disease in an Asian population. BMC Nephrol 2013; 14: 206. DOI: https://doi.org/10.1186/1471-2369-14-206
Levin GP, Robinson-Cohen C, de Boer IH, et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA 2012; 308: 1898-905. DOI: https://doi.org/10.1001/jama.2012.17304
Downloads
Published
How to Cite
Issue
Section
License
The Journal of Southeast Asian Medical Research will hold the copyright to all published articles. The publisher's production department handles copyright forms once a manuscript is accepted and scheduled for publication.







