COMPARISON OF EFFICACY IN RENOPROTECTION BETWEEN AZILSARTAN AND ENALAPRIL: A RANDOMIZED CONTROLLED TRIAL
DOI:
https://doi.org/10.55374/jseamed.v7.156Keywords:
Hypertension, Antihypertensive therapy, Azilsartan Medoxomil, Enalapril, Microalbuminuria, Macroalbuminuria, Renoprotective effectAbstract
Background: Angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs) are reported to improve renal outcomes among patients with hypertension and chronic kidney disease (CKD), but there might be substantial differences in their renoprotective effects. Azilsartan medoxomil is a relatively new available ARB, highly specific angiotensin type 1 receptor and superior in terms of blood pressure reduction, with respect to other ARBs.
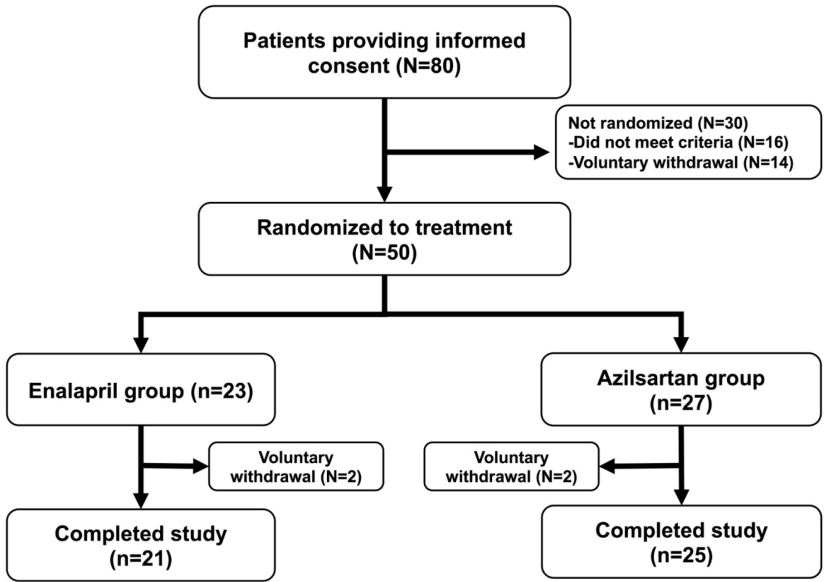
Methods: The study employed a randomized controlled trial; hypertensive subjects with albuminuria >30 mg/g creatinine at the outpatient clinic, Phramongkutklao Hospital, Bangkok, Thailand were randomly assigned to azilsartan 40-80 mg/day (n=27) or enalapril 10-40 mg/day (n=23) for 24 weeks. The primary outcome was the change in urine albumin creatinine ratio (UACR). UACR, estimated glomerular filtration rate (GFR), blood pressure and serum electrolytes were evaluated at baseline, 12 and 24 weeks.
Results: A total of 50 patients with hypertension and albuminuria were recruited. At the end of treatment, systolic blood pressure level was significantly reduced in the azilsartan group compared with the enalapril group (-12.2 mmHg [95%CI -18.9 to -5.5] vs. -1.1 mmHg [95% -7.8 to 5.7], p=0.021). In addition, at 24 weeks, significantly reduced median UACR was observed in the azilsartan group compared with that of the enalapril group (-59.9 mg/g Cr [95% CI -284.6 to -31.0] vs. -40.4 mg/gCr [95% CI -129.4 to 88.3], p=0.026)). No statistically significant difference was found between the two groups in hyperkalemia, estimated GFR, acute kidney injury and serious adverse events.
Conclusion: This study demonstrated that azilsartan had superior antihypertensive and albuminuric efficacy compared with the standard dose of enalapril without increasing adverse events.
Downloads
Metrics
References
Ames MK, Atkins CE, Pitt B. The reninangiotensin-aldosterone system and its suppression. J Vet Intern Med 2019; 33: 363-82. DOI: https://doi.org/10.1111/jvim.15454
Ichikawi I, Harris RC. Angiotensin actions in the kidney: renewed insight into the old hormone. Kidney Int 1991; 40: 583-96. DOI: https://doi.org/10.1038/ki.1991.249
Stevens PE, Levin A. Kidney Disease: Improving global outcomes chronic kidney disease guideline development work group M: Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825-30. DOI: https://doi.org/10.7326/0003-4819-158-11-201306040-00007
Hjermitslev M, Grimm DG, Wehland M, Simonsen U, Kruger M. Azilsartan Medoxomil, an Angiotensin II Receptor Antagonist for the Treatment of Hypertension. Basic Clin Pharmacol Toxicol 2017; 121: 225-33. DOI: https://doi.org/10.1111/bcpt.12800
Bönner G, Bakris GL, Ica D, Weber MA, W B White WB, Perez A, et al. Antihypertensive efficacy of the angiotensin receptor blocker azilsartan medoxomil compared with the angiotensin-converting enzyme inhibitor ramipril. J Hum Hypertens 2013; 27: 479-86. DOI: https://doi.org/10.1038/jhh.2013.6
Gitt AK, Bramlage P, Potthoff SA, Baumgart P, Mahfoud F, Hartmut Buhck H, et al. Azilsartan compared to ACE inhibitors in anti-hypertensive therapy: one-year outcomes of the observational EARLY registry. BMC Cardiovasc Disord 2016; 16: 56. DOI: https://doi.org/10.1186/s12872-016-0222-6
Suehiro T, Tsuruya K, Yoshida H, Tsujikawa H, Yamada S, Tanaka S, et al. Stronger Effect of Azilsartan on Reduction of Proteinuria Compared to Candesartan in Patients with CKD: A Randomized Crossover Trial. Kidney Blood Press Res 2021; 46: 173-84. DOI: https://doi.org/10.1159/000512365
Dargad RR, Parekh JD, Dargad RR, Kukrety S. Azilsartan: Novel Angiotensin Receptor Blocker. J Assoc Physicians India 2016; 64: 96-8.
White WB, Weber MA, Sica D, Bakris GL, Perez A, Cao C, et al. Effects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertension. Hypertension 2011; 57: 413-20. DOI: https://doi.org/10.1161/HYPERTENSIONAHA.110.163402
Hye Khan MA, Neckar J, Cummens B, Wahl GM, Imig JD. Azilsartan decreases renal and cardiovascular injury in the spontaneously hypertensive obese rat. Cardiovasc Drugs Ther 2014; 28: 313-22. DOI: https://doi.org/10.1007/s10557-014-6530-0
Hou Y, Zhang F, Liu Z, Su S, Wu X, Wang Z. Effect of telmisartan and enalapril on ventricular remodeling and kidney prognosis of patients with coronary artery disease complicated with diabetic nephropathy. Exp Ther Med 2017; 13: 131-4. DOI: https://doi.org/10.3892/etm.2016.3933
Ojima M, Igata H, Tanaka M, Sakamoto H, Kuroita T, Kohara Y, et al. In vitro antagonistic properties of a new angiotensin type 1 receptor blocker, azilsartan, in receptor binding and function studies. J Pharmacol Exp Ther 2011; 336: 801-8. DOI: https://doi.org/10.1124/jpet.110.176636
Bakris GL, Sica D, Weber M, White WB, Roberts A, Perez A, et al. The comparative effects of azilsartan medoxomil and olmesartan on ambulatory and clinic blood pressure. J Clin Hypertens (Greenwich) 2011; 13: 81-8. DOI: https://doi.org/10.1111/j.1751-7176.2010.00425.x
Rakugi H, Enya K, Sugiura K, Ikeda Y. Comparison of the efficacy and safety of azilsartan with that of candesartan cilexetil in Japanese patients with grade I-II essential hypertension: a randomized, double-blind clinical study. Hypertens Res 2012; 35: 552-58. DOI: https://doi.org/10.1038/hr.2012.8
Takami T, Okada S, Saito Y, Nishijima Y, Kobori H, Nishiyama A. Effects of Olmesartan and Azilsartan on Albuminuria and the Intrarenal Renin-Angiotensin System. World J Res Rev 2018; 6: 7-10. DOI: https://doi.org/10.31871/WJRR.6.1.18
Nakamura T, Fujiwara N, Kawagoe Y, Sugaya T, Ueda Y, Koide H. Effects of telmisartan and enalapril on renoprotection in patients with mild to moderate chronic kidney disease. Eur J Clin Invest 2010; 40: 790-6, DOI: https://doi.org/10.1111/j.1365-2362.2010.02319.x
Mani A. Albuminuria in hypertensive patients: Where the choice of antihypertensive medications matters:: Commentary on “Several conventional risk markers suggesting presence of albuminuria are weak among rural Africans with hypertension”. J Clin Hypertens (Greenwich) 2016; 18: 31-2. DOI: https://doi.org/10.1111/jch.12660
Wang K, Hu J, Luo T, Wang Y, Yang S, Qing H, et al. Effects of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on All-Cause Mortality and Renal Outcomes in Patients with Diabetes and Albuminuria: a Systematic Review and Meta-Analysis. Kidney Blood Press Res 2018; 43: 768-79. DOI: https://doi.org/10.1159/000489913
Hye Khan MA, Neckar J, Haines J, Imig JD. Azilsartan improves glycemic status and reduces kidney damage in zucker diabetic fatty rats. Am J Hypertens 2014; 27: 1087-95. DOI: https://doi.org/10.1093/ajh/hpu016
Eijkelkamp WBA, Zhang Z, Remuzzi G, Hans-Parving H, CooperME, Keane WF, et al. Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol 2007; 18: 1540-6. DOI: https://doi.org/10.1681/ASN.2006050445
Downloads
Published
How to Cite
Issue
Section
License
The Journal of Southeast Asian Medical Research will hold the copyright to all published articles. The publisher's production department handles copyright forms once a manuscript is accepted and scheduled for publication.







